
Let’s talk specs and logistics. You’re not just moving boxes of hyaluronic acid filler; you’re supplying a critical medical device that architects faces and builds businesses worldwide. For distributors and importers, understanding the product’s journey from our manufacturing line to the practitioner’s needle is non-negotiable. It’s what separates a commodity supplier from a strategic partner. Here’s the raw, operational breakdown.
The Global Demand Landscape: Where Volume Meets Precision
اعداد دروغ نمی گویند. بازار جهانی پرکننده های پوستی فقط در حال رشد نیست; it’s segmenting and sophisticating at a pace that demands your attention. As of this quarter, the data shows a clear divergence between established and emerging markets. North America and Europe remain volume leaders, but the growth velocity in the Asia-Pacific region, particularly South Korea, Japan, and increasingly Southeast Asia, is reshaping procurement strategies. The key for you, the B2B trader, is granularity. It’s no longer about “facial fillers.” It’s about specific product attributes: high-G’ fillers for structural contouring in the chin and jawline are seeing a 20%+ year-on-year demand spike in cosmopolitan hubs. در ضمن, low-density, hyper-diluted fillers for delicate under-eye areas are the fastest-moving SKU in markets where subtle, “preventative” aesthetics dominate. Your inventory must reflect this clinic-floor reality.
| منطقه | Dominant Application Trend (2024) | CAGR پیش بینی شده (2024-2029) | Key B2B Consideration |
|---|---|---|---|
| آمریکای شمالی | High-Viscosity Fillers for Cheek & Jaw Augmentation | 8.2% | Demand for comprehensive procedural kits, including cannulas. |
| اروپای غربی | Mid-Density Fillers for Nasolabial Folds & Lip Balance | 7.1% | Stringent CE-MDR compliance documentation is a non-negotiable for clearance. |
| آسیا و اقیانوسیه | Micro-Droplet Techniques for Tear Trough & هیدراتاسیون | 14.5% | Preference for prefilled, ultra-fine gauge syringes (32G+). |
| آمریکای لاتین | Combination Therapies (Filler + Biostimulant) | 9.8% | Need for dual-supply chains and bundled product education. |
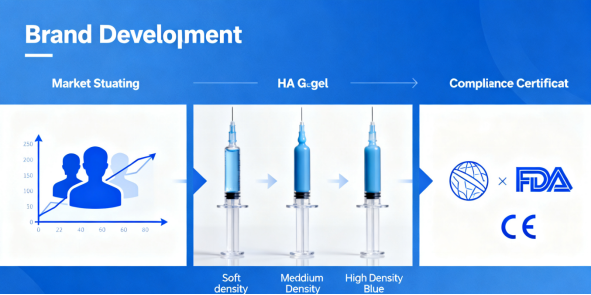
Decoding the Technical Dossier: Beyond Hyaluronic Acid Concentration
Your clients—clinics and medspas—are getting smarter. They’re asking about cross-linking technology, not just HA concentration. When you’re fielding inquiries, here’s what you need to articulate. The product’s performance is dictated by three pillars: the raw HA source (bacterial fermentation is the gold standard for purity and consistency), the cross-linking agent (BDDE remains dominant, but its precise ratio and quenching process define safety profiles), and particle uniformity. A monophasic, cohesive polydensity filler offers predictable tissue integration and smoother injection feel, a major selling point for practitioners new to the brand. Be ready to provide the Certificate of Analysis that details residual BDDE levels (به طور معمول <2 ppm for premium lines), sterility testing, and endotoxin limits. This paperwork is your passport through customs and into reputable clinics.
The Practitioner’s Protocol: A Logistics-First View of Application
Think of each syringe as a precision tool with a strict operational manual. برای B2B, “how to use” translates to “how to ensure optimal outcomes in the field to minimize returns and build re-orders.” The chain is critical. اول, Storage & Transport: Maintain 2-8°C from our warehouse to your hub and, ideally, to the end clinic. Freezing is a deal-breaker—it creates ice crystals that disrupt the gel matrix. دوم, Pre-Use Handling: The product must be warmed to room temperature naturally (no water baths) برای 30 minutes before use. A cold filler is viscous, increases injection pain, and can lead to clumping. سوم, Injection Dynamics: This is your value-add. Educate your clients that filler from our line is engineered for low extrusion force. It pairs optimally with 27G-30G needles for sharp needle techniques or 25G-27G blunt cannulas for safer plane dissection. The product’s flow characteristics reduce hand fatigue during multi-syringe sessions, a tangible benefit for high-volume practices.
Managing the Cold Chain & Regulatory Pipeline
Your operational backbone is compliance and logistics. Different markets, different rules. For the EU, you need a full technical file under MDR. For the USA, it’s a PMA or 510(ک) clearance held by the manufacturer, but you as the importer of record have strict FDA registration and listing obligations. For emerging markets in the Middle East or Latin America, local health ministry approvals often require in-country testing and specific labeling in the local language. The filler itself is thermally stable for up to 24 months at 2-8°C, but shipping delays or customs holds can eat into that shelf life. Our recommendation? Implement a first-expiry-first-out (FEFO) inventory system and consider bonded warehousing in strategic re-export hubs like Dubai or Singapore to serve multiple regions with agility.
FAQs for the Global Distributor
س: What is the minimum order quantity (MOQ) for a new distributor, and can we mix SKUs?
الف: Our standard MOQ is 500 units per SKU for initial orders to ensure commercial viability. با این حال, we actively support portfolio diversification. We offer mixed pallet options after the first order, allowing you to combine high-G’, mid-density, and lip-specific fillers to meet varied clinic needs. We provide consolidated documentation to simplify customs clearance for mixed shipments.
س: How do you support us with clinical training for our end-users?
الف: We provide a full suite of B2B2C materials: digital Masterclass modules on injection techniques specific to our product’s rheology, 3D anatomy software licenses for patient consultation, and access to live procedural workshops in key cities. We also supply disposable demonstration kits with practice gels for hands-on training events you host.
س: Can you provide real-time shipment tracking and stability data logs?
الف: بله. Each shipment is equipped with a Bluetooth temperature logger. You and your end-client can access the complete temperature history via a secure cloud portal. This document serves as proof of integrity throughout the cold chain and is essential for liability management and quality assurance audits.
س: What is your policy on damaged goods or suspected cold chain breaches?
الف: We mandate immediate photographic documentation of the received goods and the temperature logger data. Our insurance covers replacements for shipments where a breach (temperature excursion beyond 2-8°C for >60 minutes) is verifiable via the data log. Claims must be initiated within 48 hours of delivery. We manage this process swiftly to protect your inventory and client trust.
