What You’re Really Selling: The Science and Specifications of Modern HA Fillers

Let’s cut straight to the point. As a B2B distributor, you’re not just moving vials of gel; you’re supplying a precise biomechanical tool. The core of what makes one hyaluronic acid (HA) filler different from another boils down to its physicochemical properties: cross-linking technology, HA concentration, particle size, and G-prime (gel stiffness). These aren’t just specs on a datasheet; they directly dictate clinical application and, ultimately, your customer satisfaction.
Think of it this way. A high G-prime, cohesive gel is your workhorse for deep structural volumizing—cheekbones, chins, and jawline contouring. It’s strong, holds its shape under tissue pressure, and provides that “scaffolding” effect. On the other hand, a low G-prime, more fluid filler is designed for fine lines, lip enhancement, and superficial dermal hydrating injections. It integrates softly for a natural feel. The cross-linking density (using agents like BDDE) determines how long the product lasts in the skin by resisting enzymatic breakdown. More cross-linking typically equals longer longevity but requires advanced proprietary manufacturing to keep it smooth and non-immunogenic.
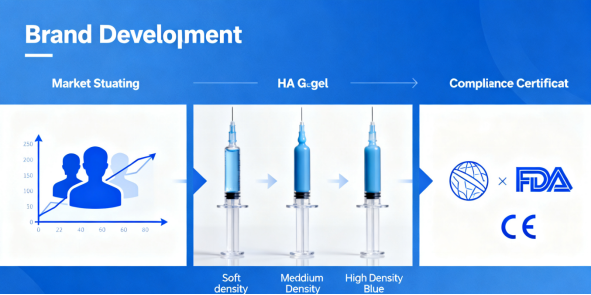
For you, this means your clients—clinics and medspas—need a curated portfolio. They might need 2-3 different types of fillers from your line to treat a full face. Your ability to explain why “Filler A” is for cheeks and “Filler B” is for lips, based on this hard data, positions you as a technical partner, not just a vendor.
Market Pulse: The Global Demand Driving Your Supply Chain
The numbers don’t lie, and for a distributor, they are your roadmap. The global dermal filler market isn’t just growing; it’s accelerating, driven by factors you can bank on.
| Region | Projected CAGR (2024-2029) | Key Drivers | Prime Product Focus |
|---|---|---|---|
| North America | ~9.5% | High disposable income, preventative aging, strong medspa culture. | Premium, high-longevity fillers, combination treatments. |
| Asia-Pacific | ~13.5% (Leader) | Exploding medical tourism, rising beauty consciousness, tech adoption. | Versatile fillers for facial contouring, subtle enhancement. |
| Europe | ~8.8% | Established regulatory framework, demand for natural results. | Highly documented, safety-focused products with CE marking. |
| Middle East | ~11% | High-volume procedures, defined aesthetic preferences. | Fillers for strong contouring and volumizing. |
Real-time insight? Look beyond traditional aesthetics. The “tweakment” trend (subtle, regular maintenance) favors softer, more natural-looking HA products. Meanwhile, the medical application segment—scar revision, osteoarthritis joint injection (like for knees), and even wound healing—is a burgeoning, less-saturated channel with different regulatory and purchasing pathways. Distributors who recognize these dual channels (aesthetic vs. therapeutic) unlock new revenue streams.
From Shelf to Syringe: Applications That Close Deals
You need to equip your sales team with concrete “how-to-sell” knowledge. Here’s where application expertise translates to order volume.
For facial contouring and volumizing, the game is about 3D modeling. It’s not just filling wrinkles; it’s restoring age-related volume loss in the mid-face (cheeks, temples). This requires those high G-prime fillers we talked about. The technique is key—deep supraperiosteal injection for a lifting effect. Your clinical training support on this is a major value-add.
Lip enhancement remains a top request, but trends have shifted from the overfilled “duck lip” to a more natural, defined shape with subtle volume and vertical lip lines treatment. This requires a medium-to-low G-prime filler with excellent smoothness for seamless integration.
The real growth area? Bio-rejuvenation and skin quality. Techniques like micro-droplet HA injection (mesotherapy) or using diluted HA to improve skin hydration, elasticity, and luminosity are hugely popular. This positions HA filler as a “skin health” product, appealing to a broader, perhaps younger, clientele for your clinics. It also increases the volume of filler used per treatment session.
The Non-Negotiables: Safety, Compliance, and Your Liability
This is the critical backbone of your business. For B2B, selling a medical device means sharing responsibility for its safe deployment. Your manufacturers must provide not just CE marks or FDA approvals, but full technical dossiers, certificates of analysis (CoA) for every batch, and clear instructions for use (IFU).
The big topic now is Radiesse Compatibility. With the rise of calcium hydroxylapatite (CaHA) fillers like Radiesse, there’s a clinical technique of layering HA over CaHA for a combined effect. Your product data must explicitly state compatibility and safety for such use. Any ambiguity here is a deal-breaker for sophisticated clinics.
Viscosity, extrusion force, and needle gauge compatibility are your operational concerns. A filler that’s too viscous might be difficult to inject through a fine-gauge needle, affecting practitioner comfort and precision. You need to test and validate these parameters in real-world conditions.
Q&A: Your Top Technical and Commercial Questions Answered
-
Q: With so many manufacturers, how do we verify the quality and purity of the HA raw material?
A: Demand transparency. Top-tier manufacturers use pharmaceutical-grade, non-animal sourced HA from biofermentation. They should provide documentation on the HA source, molecular weight distribution, and most critically, residual cross-linker (BDDE) levels, which should be far below the stringent limits set by pharmacopoeias (like EP/USP). This is directly linked to biocompatibility and low swelling risk. -
Q: We see “monophasic” and “biphasic” fillers. What’s the practical difference for our clinic clients?
A: Monophasic gels are a single, homogeneous phase of cross-linked HA. They tend to be smoother, more cohesive, and are often preferred for areas requiring softness and natural integration (lips, tear troughs). Biphasic gels contain cross-linked HA particles suspended in a non-cross-linked HA gel. They can offer a robust volumizing effect and are often used for deeper implantation. The choice isn’t about “better,” but about clinical indication and practitioner preference. -
Q: How critical is the packaging and delivery system to our clients?
A: Extremely. It’s a major part of the user experience. Pre-filled syringes with low extrusion force reduce hand fatigue for practitioners. Clear, precise scale markings are essential for accurate dosing. Luer-lock compatibility is standard. Some leading systems now offer integrated needle designs or ultra-fine gauge options (like 33G) for virtually painless injections, which is a powerful selling point for clinics focusing on patient comfort. - Q: What does “latest generation” cross-linking actually mean, and why should we pay a premium for it?
A: “Latest generation” refers to advanced cross-linking and purification processes (e.g., Optimal Balance Technology, various proprietary names). The goal is to maximize the durability of the product while minimizing chemical residues and creating a more natural, malleable gel. This results in fillers that last longer (12-24 months), integrate more smoothly with less swelling, and have an outstanding safety profile. For distributors, this means fewer complications for your clients and a stronger brand reputation that justifies a higher price point.
